Copyright © 2022 Binhui Bio All Rights Reserved
鄂ICP备16007335号-1Legal Terms
Partners
Quality Management
System
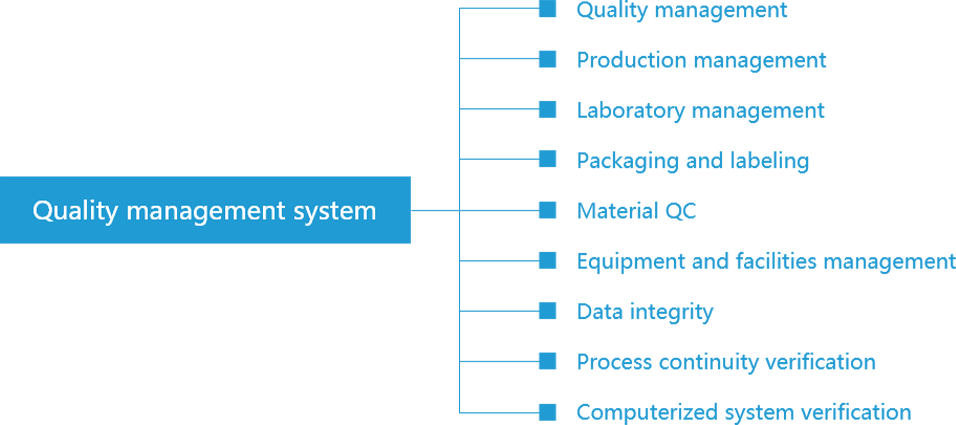
The company adheres to the "quality-directed, regulations-centered, specialists-managed, end-to-end-controlled" quality control policy and follows the latest version of <Guide to Good Manufacturing Practice for Medicinal Products>. The company has established and will continuously improve the quality management system in seven dimensions including quality risk, document, quality control, clinical trial, product monitoring and authentication system management. It allows the quality assurance team to conduct deep supervision and management of R&D, production, test, determination, release, pharmacovigilance, complaints, and other stages in the process of system operation, while the quality control team can keep their independence and authority. The company's quality management system keeps optimization through benchmarking with the current relevant laws and regulations of different countries and importing advanced international quality concepts.
The company has been implementing the quality risk management policy of "integrity, quality, responsibility, and benefit" and applying its philosophy to various quality modules such as deviation management, change control, corrective and preventive measures, and environmental monitoring, to ensure that routine production supervision and quality control risks are fully controllable from the source, and the high-quality consistency of the products. Thanks to efforts of all employees, the company has established an effective quality management system.

